Critical Limb Ischemia
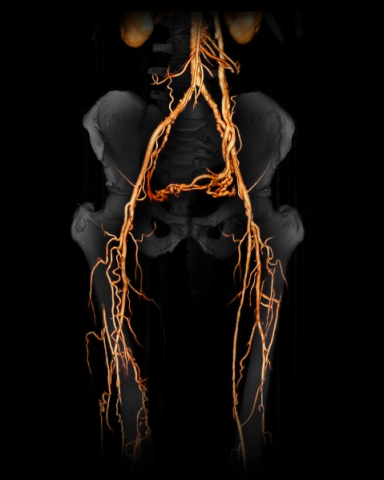
CLI - BD: Critical Limb Ischemia due to Buerger's Disease,
CLI - PAD: Critical Limb Ischemia due to atherosclerotic Peripheral Artery Disease
CLI is the most serious form of Peripheral Arterial Disease (PAD). PAD patients have severe blockage of the arteries of the lower extremities, leading to markedly reduced blood-flow and ulceration, often leading to lower-limb amputation. Worldwide there are about 25 million patients affected with CLI, yet there are no effective therapies. Mortality rates are very high - the 5 year mortality rate is 67%. Overall, approximately 40% to 50% of CLI patients will lose their leg within 6 – 12 months and approximately 15% will require contralateral amputation within 2 years.
Stempeucel® is a first of its kind allogeneic, MSC therapy. It is produced by pooling bone marrow-derived MSC's of healthy individuals through a proprietary, patented process. Research conducted at Stempeutics has shown that pooling balances out variations observed with individual donor cells, resulting in a product with strong immune-modulatory properties, broader cytokine / growth factors array, longer lifespan and consistent clinical outcomes. Pooling underpins the clinical success demonstrated by Stempeucel®. Stempeucel® works thru' anti-inflammatory & immuno-modulatory properties and by inducing angiogenesis in ischemic muscle – ultimately leading to improvement in all the clinically relevant endpoints for CLI
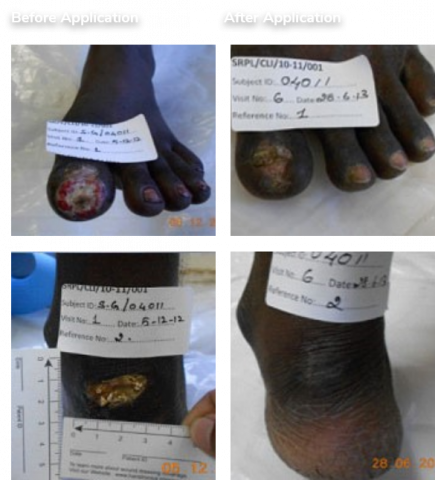
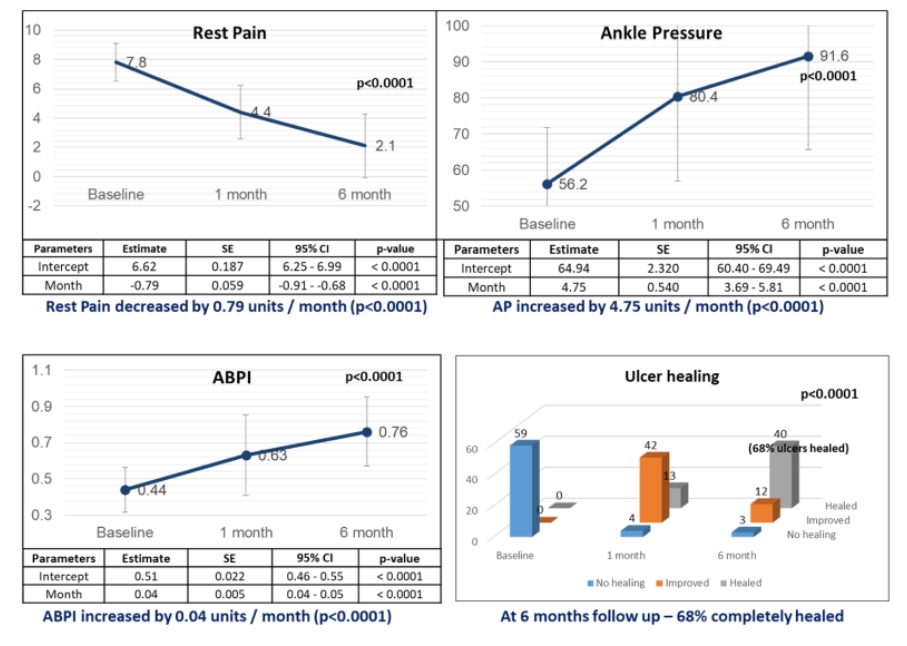
Positive Phase 4/PMS study data in terms of efficacy & safety for CLI-BD. Positive Phase 3 study data in terms of efficacy & safety for CLI-PAD
Phase 4/PMS study data for CLI BD is also consistent with Phase 2 data conducted earlier as shown in the graphs.
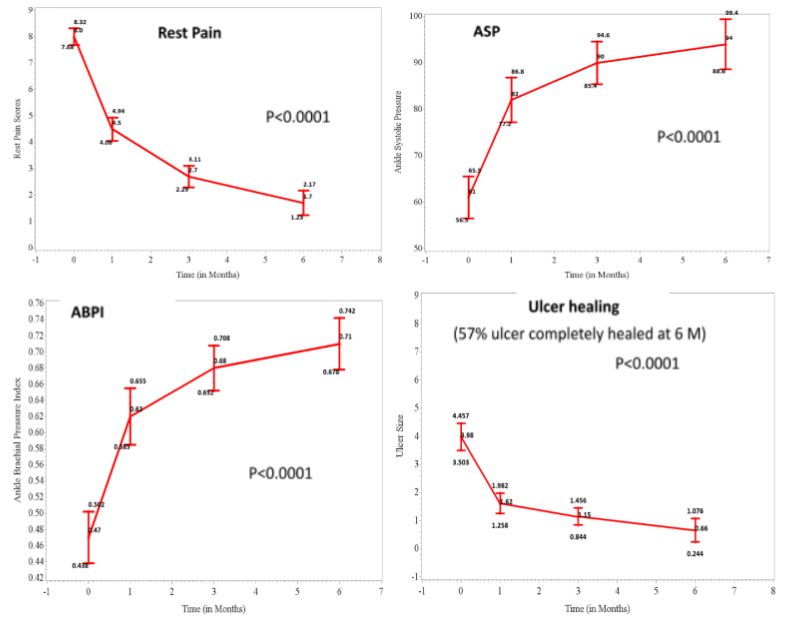
Phase 3 label extension study for CLI PAD is also consistent with the CLI BD data:
Stempeucel® offers significant benefits for vascular as well as other regenerative diseases, and has the potential to become a global blockbuster therapy that provides patients with a much needed therapeutic benefit. With no other allogeneic pooled MSC therapy in development, Stempeucel® is expected to re-define the treatment landscape for CLI.
Stempeutics is currently seeking partnership for the development and commercialization of Stempeucel® to treat CLI in markets outside India.
The opportunity is significantly de-risked due to:
- Positive results from a large Phase 2 trial that demonstrated efficacy & safety
- Positive Phase 4/PMS study data in terms of efficacy & safety for CLI-BD
- Positive Phase 3 study data in terms of efficacy & safety for CLI-PAD
- Marketing approval granted in India for CLI-BD & CLI-PAD
- Patent-protected pooling technology provides unique differentiation by reducing the inter-donor and inter-batch variations compared to other allogeneic stem cell products in the pipeline, whilst providing defensibility against the competition