Knee Osteoarthritis
Take Steps Toward Better Joint Health
Speak to an expert to understand your condition and a suggested course of action.
Stempeucel® can reduce joint pain via anti-inflammatory effects, and possess chondrogenic differentiation potential In addition, Stempeucel® could provide signal to resident chondro-progenitors of the host to differentiate into chondroblasts and further into chondrocytes, to repair cartilage damage
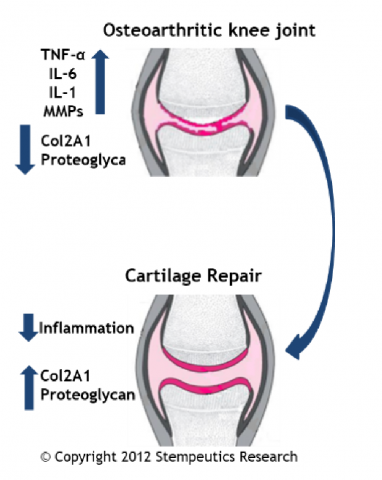
Randomized, double blind, Phase 3, multi-centric, Placebo controlled study assessing the efficacy & safety of IA administration of stempeucel®
To assess the change from baseline to one year in WOMAC (Western Ontario and McMaster Universities Osteoarthritis) Osteoarthritis Composite Index score as compared to the placebo arm
To assess the change from baseline to one year and two years follow-up as compared to the placebo arm in
- WOMAC OA Pain Index/ Stiffness Index / Physical function Index
- Patient's Assessment of Osteoarthritis Pain by VAS
- MRI imaging (done at baseline, 6, 12 & 24 months) to evaluate:
- Assess the cartilage quality by T2 mapping
- Assess the cartilage morphology
- Assess the cartilage volume
Assessment of AE(s) + ECG parameters/vital signs
- Assessment of biomarkers: CTX – II (urine)
- Assessment of antibodies: Anti HLA antibody
Product Development Status
Current status of Clinical Trials: All clinical trials are approved by DCGI in India