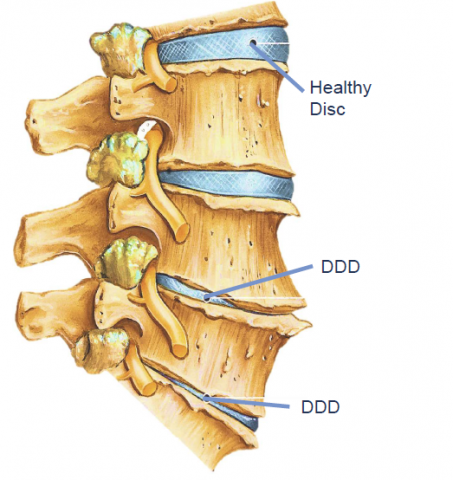
Stempeucel® Chronic Low Back Pain
Disease Overview
Image
STEMPEUCEL®
Mechanism of Action of Stempeucel®
Image
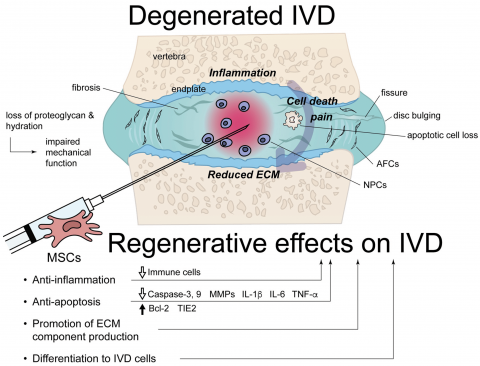
MOA of MSCs in CLBP
- Anti-inflammatory
- Anti-apoptotic
- Anti-pyroptotic
- Promotion of ECM production
- Increase in the number of IVD cells (NPC / AFC) due to MSC differentiation
- Reduced disc tissue degeneration,
- Recovery of disc height &
- Decreased pain
Only 27% of patients could return to work after 2 years of fusion surgery
Surgery model: Disc Puncture Model in Sprague Dawley Rat
Major findings:
- Highest dose tested attenuated the traumatic IVDD-induced allodynia
- There was marked recovery of gait
- Recovery in histological features and chrondogenic markers
- Hence, pre-clinical studies showed evidence of regeneration of the disc after local administration of stempeucel®
- Plan to go ahead with phase 2 dose finding clinical trial
OUR PROGRESS
Product Development Status
Chronic Low Back Pain
Indication
Basic R&D
Pre-Clinical
Phase 1
Phase 2
Phase 3
Marketing Authorization
Current status of Clinical Trials: All clinical trials are approved by DCGI in India