COVID-19 ARDS
Disease Overview
Image
Image
Management of ARDS
Image
While there are many medicines available to treat mild to moderate cases of COVID-19 patients, for treating Covid-19 ARDS patients, only limited options are available which includes ICU admission, high flow nasal canula oxygen therapy/ invasive or noninvasive ventilation and treatment which includes steroids, anti-coagulants and supportive measures to maintain euvolemia and to manage any complications.
Image
Patients with ARDS that are ventilated for an extended period of time and survive frequently experience long term pulmonary damage due to ventilator induced fibrosis and scarring, and also experience compromised quality of life.
Image
The current therapeutic interventions do not appear to be improving in-hospital survival. The mortality rate is high (>39%).Hence, there is a large unmet need for a safe and effective treatment for COVID-19 infected patients, especially the severe cases complicated with ARDS.
STEMPEUCEL®
Mechanism of Action of Stempeucel®
It is envisaged, Stempeucel® will reduce the fatal symptoms of COVID 19 induced pneumonia and progressing to ARDS
Image
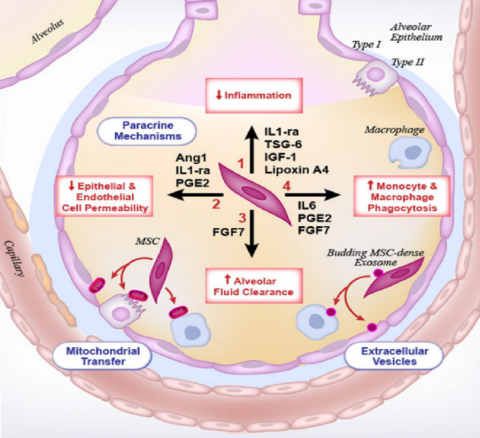
It is envisaged, Stempeucel® will reduce the fatal symptoms of COVID-19 induced pneumonia and progressing to ARDS
- Paracrine factors that modulate tissue repairs
- Anti - inflammatory effects on host cells
- Reduction of alveolar epithelium permeability in the lung
- Increased rate of alveolar fluid clearance
- Enhancement of host mononuclear cell phagocytic activity
- MSCs directly transfer mitochondrial DNA to host cells
- MSCs secrete micro vesicles
- MSCs resistant to viral infection by IFN stimulated genes
Clinical Trial Design
Phase 3 Study Assessing the Efficacy and Safety of IV administration of Stempeucel® in Patients with Moderate to Severe ARDS due to COVID 19
Percent mortality 28 days after administration of BMMSCs
To assess the change from baseline to one year and two years follow-up as compared to the placebo arm in
- ICU free days 28 days after administration of BMMSCs
- Number of days alive and off ventilatory support at 28 days after administration of BMMSCs
- Oxygen index (OI) changes (from day 0 to day 14) (Oxygen index = FiO2 x MAP x 100 / PaO2)
- Acute Physiology And Chronic Health Evaluation (APACHE) II score (from Day 0 to Day 14)
- Sequential organ failure assessment score (SOFA score) (at day 0, 1, 3,7,14,and 28)
- Sequential organ failure assessment score (SOFA score) (at day 0, 1, 3,7,14,and 28)
- The type of adverse events AE(s), number of AE(s) and proportion of patients with AE(s).
- Assessment of clinical laboratory parameters.
- Physical examination findings and assessment of vital signs.
- Assessment of electrocardiogram (ECG) parameters.
- Plasma cytokines – IL -6, IL-8 and IL-10 (at day 0, day 4, 7, 14 and 28)
- Other markers in plasma – TNFα, D-dimer, LDH, hs-troponin, Pro-calcitonin, CRP (at day 0, 4, 7, 14 and 28)
OUR PROGRESS
Product Development Status
ARDS due to COVID-19 Pneumonia
Indication
Basic R&D
Pre-Clinical
Phase 1
Phase 2
Phase 3
Marketing Authorization
Current status of Clinical Trials: All clinical trials are approved by DCGI in India